Lab 2: Aluminum Foil Lab
Aluminum Foil Lab
Ben Fredeen
Purpose:
The purpose of this experiment was to calculate the height of a sheet of aluminum foil in millimeters without measuring it with a ruler. To do this you use the density and mass find the volume and then use the length and width to find the height. The important terms for this lab are volume, mass, density and significant digits. Volume is the amount of space an object takes up or the mass divided by density. Density is the how compact and object is or the mass divided by volume. Mass is the amount of matter in an object or the volume times density. Significant digits are all of the digit read plus one estimated digit when measuring.
Procedure:
To begin this lab, we first took a piece of aluminum foil with the density of 2.7 g/cm3. Then, we measured each side of the foil using a 30 cm ruler, with marked millimeters, and found the length and width which were 10.45cm and 9.97cm respectively. When measuring we made sure that our estimates had only one estimated digit after the certain digits. Then, we placed the piece of aluminum foil on the scale and found the mass which was 0.4g. Since we knew both the mass and density, we were able to calculate the volume by dividing the mass by density which was 0.4g / 2.7g/cm3 = 0.148cm3 which would be 0.1 cm3 when following the significant digit rules. Since the volume is equal to the length width and height multiplied together, we divided the volume by the quantity of the length times width which was 0.1cm3/ (10.45cm x 9.97cm) = 0.000959817cm which is rounded to 0.0001cm using the significant digit rules. To convert this value from centimeters to millimeters, all we needed to do was multiply by ten because there are ten millimeters in a centimeter so, 0.0001cm x 10 = 0.001mm.
Data:
First, I calculated the volume by dividing the mass by density which was 0.4g / 2.70g/cm3 = 0.148148cm3 = 0.1cm3. Then, I divided the volume by the quantity of the length times width because the length times width times height equals the volume. This was, 0.1cm3 / (10.45cm x 9.97cm) = 0.000959817 cm = 0.001 cm. After, I converted the result in centimeters to millimeters by multiplying by 10 so it was 0.001 cm x 10 = 0.01 mm.
Conclusions:
The surprising part of the this lab to me was how small the height of aluminum foil was and how easy it is to calculate given just a few pieces of information like the mass or density. This surprised me because it seems like it would be a lot more complex and hard to find, but it was really just a few simple equation with long decimals that were rounded. Also, I still can't believe how thin the foil really is because compared to paper, it appears much thicker are easy to see. The challenging thing about this lab was probably correctly estimating the measurement of the length and width and making sure they were close to exact.



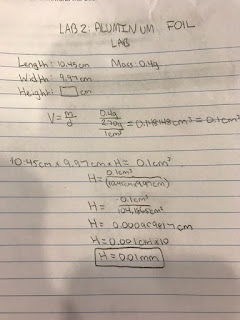



Comments
Post a Comment